Medical Device Testing
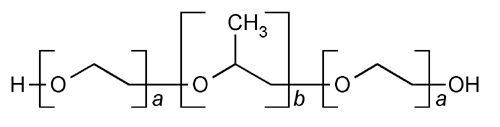
Poloxamer, also known as poloxalene and Pluronic, is a family of synthetic block copolymers of ethylene oxide and propylene oxide.
It is available in multiple molecular weights and various comonomer ratios.
Approaches
The Poloxamer USP Monograph calls for the following: Fourier transform infrared (FTIR) spectroscopy is used to identify the polymer. Molecular weight is determined by titration of hydroxyl endgroups. Molecular weight can also be determined by Gel Permeation Chromatography (GPC). Comonomer content (ethylene oxide to propylene oxide) is determined by Nuclear Magnetic Resonance Spectroscopy (NMR). Heavy metals content is determined by USP <231> or USP <233>. Limit of free ethylene oxide, propylene oxide, and 1,4 dioxane is determined by Gas Chromatography (GC). pH is determined potentiometrically.
Sample Considerations
The required amount of sample will vary depending on whether all tests are required. Please contact us to discuss your needs.
Experience
We have many years of experience testing to monographs as well as performing custom methods.
