Medical Device Testing
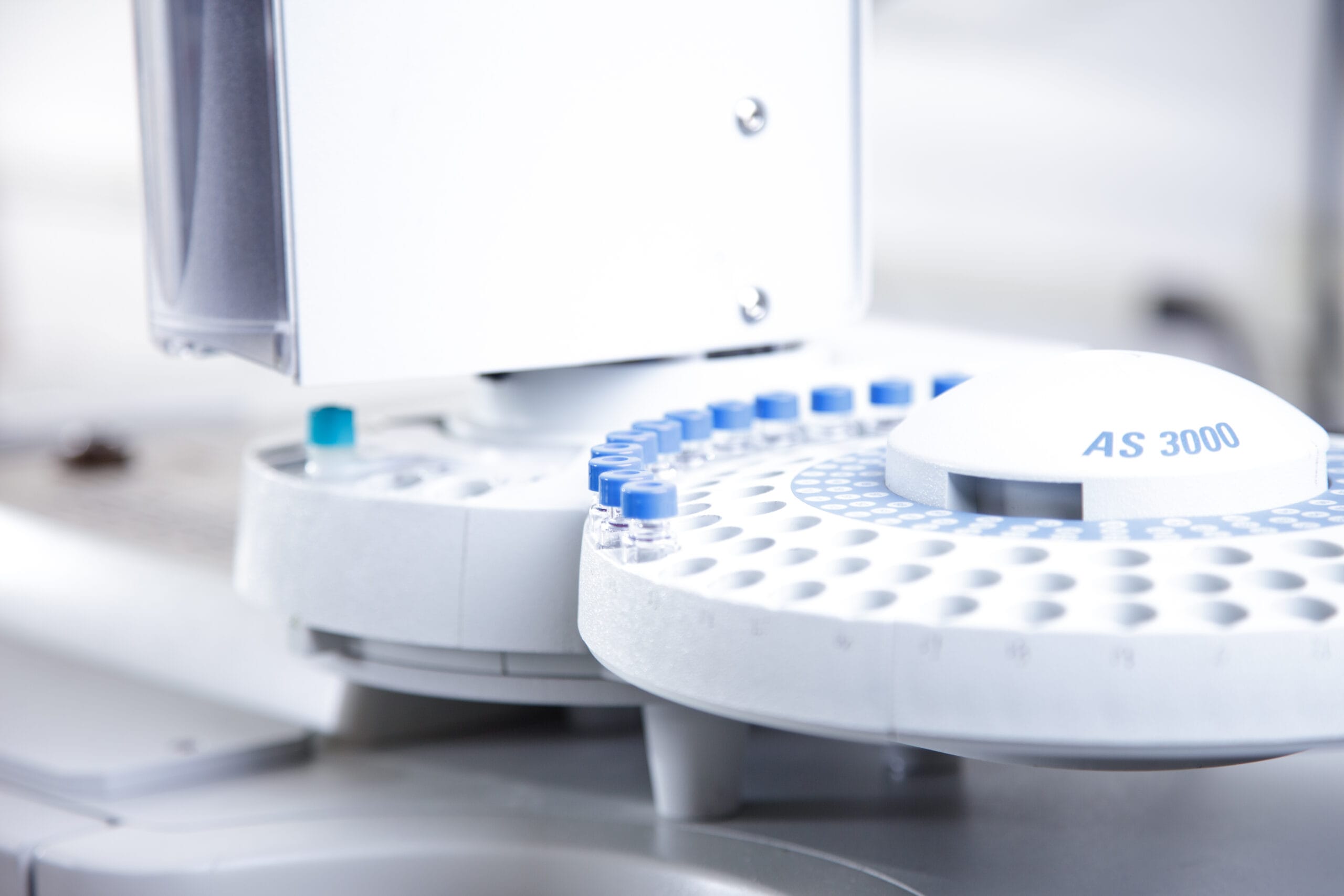
Our scientists can quantify volatile solvents in a polymer matrix through the use of Headspace Gas Chromatography/Mass Spectrometry.
Residual solvent analysis is performed through Headspace Gas Chromatography/Mass Spectrometry (Headspace GC/MS) analysis. This technique quantifies volatile solvents in a polymer matrix. A sample is placed in a closed sampling vessel and heated using a defined temperature profile. The vapor in the vessel is then analyzed.
Approaches
The sample is weighed into a headspace vial.
The sealed headspace vials are heated and agitated to drive volatiles from the solid or liquid phase into the gas phase. Sampling of the headspace for GC/MS injection is usually performed using a heated gas-tight syringe.
Sample Considerations
Virtually any sample that can be made to fit into a headspace vial can be tested. However, the sample matrix itself should not be highly volatile. Samples for quantitative residual solvent analysis should be homogeneous with respect to the concentration of volatiles.
The required sample size for residual solvent analysis is larger than the typical amount required by headspace GC/MS. A sample size of 1 g to 10 g is typical for this type of analysis.
Contact us to discuss your specific sample considerations and Residual Solvent Analysis needs.
Experience
Some examples of our experience with Residual Solvent Analysis include:
- Identification and quantification of residual solvents in packaging materials for food contact or pharmaceutical applications
- Identification and quantification of volatile organic compounds (VOC’s) present in polymers used for medical implants
- Residual solvents in pharmaceutical actives and excipients
